Author: Taha Rashid
Author: Alan Ebringer
Abstract: Ankylosing spondylitis (AS) and Crohn’s disease (CD), especially when associated with spondylitis are interrelated conditions included within the categories of spondyloarthropathic disease entities. They share some common clinical, genetic, and microbiological findings. An extensive amount of studies which have been carried out by various independent groups throughout the world have shown that Klebsiella pneumoniaemicroorganisms could be suggested as the most likely etiopathogenetic triggers for AS and CD based on the molecular mimicry mechanism and the existence of the evidence for immunological, microbiological, and molecular link between Klebsiella and self antigens. It is proposed that the use of low starch diet in conjunction with the currently used treatment might help in the eradication of Klebsiella microbes from the bowel and could result in the stoppage and alleviation of the disease process in patients with AS and/or CD.
Introduction
Ankylosing spondylitis (AS) and Crohn’s disease (CD) are among a group of related conditions which share some overlapping features, such as asymmetric oligoarthritis, inflammatory back pain, or spondylitis/sacroiliitis, enthesitis (inflammation of the entheses — the sites where the tendons or ligaments insert into the bone), anterior uveitis, a positive family history, and association with HLA-B27genetic markers, but without a positive rheumatoid factor.
These diseases are all embraced under the name of spondyloarthropathies (SpAs) (Braun and Sieper, 2010), and mainly include ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis (ReA), undifferentiated SpA (U-SpA), and enteropathic arthropathy or inflammatory bowel disease (IBD) which comprises Crohn’s disease (CD) and ulcerative colitis (UC). Apart from PsA, there is extensive evidence for the association of gut inflammation in all disease entities of SpAs to a greater or lesser extent (Mielants et al., 2005).
The Link Between HLA-B27 and Gut-mediated Arthritic Diseases
The association of HLA-B27 with AS is amongst the strongest genetic link with any common disease which has been encountered in the field of rheumatology (Thomas and Brown, 2010). This genetic bond was discovered in early 1970s, where more than 95% of patients with AS have been found to possess HLA-B27 alleles, whilst the frequency of this gene in the general population was below 10% (Brewerton et al., 1973; Schlosstein et al., 1973). Other diseases in the SpA group have lower but different degrees of associations with this allelotype (Figure 1), depending on the clinical presentation and pathological location of the disease (Braun and Sieper, 2010). For example, the frequency of this allelotype in patients with IBD/CD without associated arthritis is comparable to those of the normal population but increases to 40-60% in those patients with spondylitis/sacroiliitis. The frequency of HLA-B27 in patients with ReA/Reiter’s syndrome and U-SpA is ranging between 30 to 90% and 50 to 70%, respectively, meanwhile its frequency in patients with PsA with or without peripheral arthritis is around 20%, but it is increased to up to 60% in patients with associated sacroiliitis.
From these data results, it appears that a spondyloarthropathic patient presenting with spinal involvement had a higher chance of possessing HLA-B27 genes than those with peripheral joints involvement only. Whether patients with spinal involvements are more likely to encounter exposure to enteropathogenic bacteria and develop the disease needs further clarification.
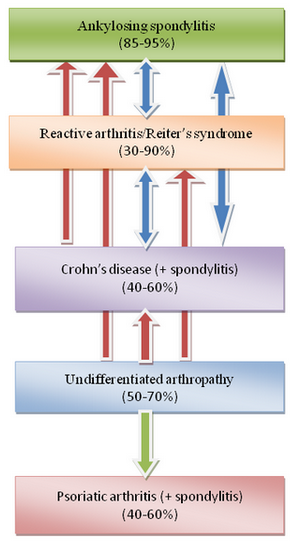
Evidence for Associated Gut Inflammation and Interrelations Between Different Disease Entities of SpAs
Many data support the existence of an inter-connection or inter-relation between different disease entities in the SpAs group. For example:
1). The prevalence of AS in patients with UC and CD was found to be reaching 2.6% and 6%, respectively, giving an overall 3.7% prevalence in patients with IBD (Palm et al., 2002). It has also been reported that AS is frequently associated with IBD, with 5-10% of cases having clinical IBD and approximately 70% of cases having subclinical bowel inflammation (Thomas and Brown, 2010), and this link was emphasized previously through an analytical review (Ebringer et al., 2007). Furthermore, HLA-B27 positive patients with IBD were shown to have higher chances of developing AS when compared to those without IBD (Wright, 1978).
2). Many studies have shown macroscopical and/or microscopical features of gut mucosal inflammation, especially in patients with IBD, ReA, AS, and U-SpA (Porzio et al., 1997; Mielants et al., 2005). In one particular study, gut changes varying from an acute to asymptomatic chronic intestinal inflammation have been observed in about 60% of patients with SpA (Demetter et al., 2002), and more interestingly, those patients who showed signs of articular remissions were preceded by disappearance of the gut inflammation, which supports the concept that the gut is involved in the pathogenesis of SpAs.
3). Another fundamental support for the role of gut and the intestinal flora in the development of SpAs in relation to the presence of HLA-B27 genes is the absence of arthritis and colitis in germ-free HLA-B27-transgenic rats and the induction of these features when the rats were relocated to non germ-free environment (Hammer et al., 1990).
4). Twenty to 30 percent of patients with unclassified HLA-B27-positive inflammatory rheumatic diseases (Sany et al., 1980) or oligoarthritis (Schattenkirchner and Kruger, 1987) have been shown to develop into one form of the definite spondyloarthropathic group such as IBD, ReA, or AS (Figure 1).
5). It has also been stated that more than half of patients with U-SpA will develop AS over a certain period of time (Mau et al., 1988). In a later study, however, no significant differences were observed in some clinico-radiological or genetic features of AS and U-SpA among the Middle Eastern and South Asian populations (Uppal et al., 2006).
It appears from these results that both HLA-B27 and gut inflammation play a pivotal role in the development of SpAs, especially AS and CD, and that the main etiopathogenetic process is triggered by a combined genetic and environmental (mainly microbial) factors.
Evidence for the Microbial Link with AS and CD
The first evidence of the epidemiological link between microbes and SpAs was detected in the early Twentieth Century, where a triad of urethritis, conjunctivitis, and arthritis, being termed as Reiter’s syndrome, was found to follow a dysenteric or venereal infection (Calin, 1998). Reiter’s syndrome was later recognized as a form of ReA and since then each of the triggering bacterial agents including Yersinia, Campylobacter, Shigella, and Salmonella enterogenic bacteria as well as Chlamydiaurogenital pathogens has been found to have an approximately equal role in the development of this disease (Leirisalo-Repo, 2005; Townes, 2010).
Although epidemiological evidence for the involvement of microbial agents in other disease entities of SpAs are lacking, a considerable degree of molecular, immunological, as well as microbiological data are available to support the role of Klebsiella pneumoniae in the etiopathogenesis of both AS (Ebringer et al., 2011) and CD (Rashid et al., 2009). Apart from some evidence for the role of mycobacteria (Rambukkana et al., 1993), however, no other microbes have been implicated in the causation of psoriasis.
Klebsiella and Self Cross-reactive Antigens
Klebsiella microbes possess various antigens which show molecular similarity and immunological cross-reactivity with HLA-B27 or other self-antigens and these have been demonstrated in several independent studies:
Evidence for molecular mimicry (Figure 2)
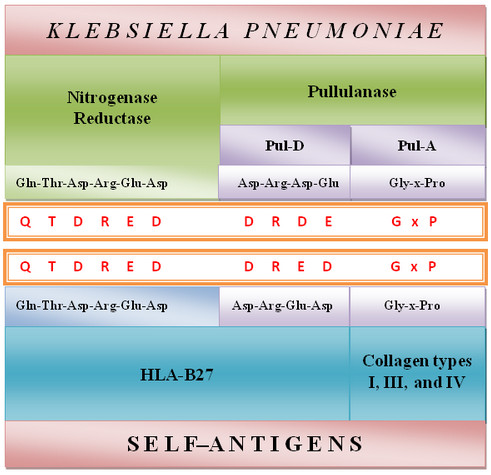
1) A molecular homology has been found between a hexameric amino acid sequence, “QTDRED” present in both HLA-B*2705 molecules (residues 72-77) and Klebsiella pneumoniae nitrogenase reductase enzymes (residues 188-193) (Schwimmbeck et al., 1989).
2) A structural similarity has been found between the “DRDE” amino acid sequences (residues 596-599) present in the Pul-D secretion protein from Klebsiella pullulanase enzyme, and the “DRED” amino acid motif (residues 74-77) present in the HLA-B27 molecules (Fielder et al., 1995).
3) Another molecular similarity composed of repeated triplet amino acid sequences “G-x-P” has been discovered between Pul-A secretion protein from Klebsiellapullulanase enzyme and collagen types I, III, and IV (Fielder et al., 1995) mainly contained in the ligaments and cartilaginous structures of spinal vertebral large joints and uvea.
Evidence for immunological cross-reactivity:
1) Antibodies obtained from a rabbit immunized with HLA-B27-positive lymphocytes showed positive reactions with the antigenic extracts of five gut-inhabited bacterial agents including Klebsiella, Enterobacter, Salmonella, Shigella, and Yersinia microbes indicating the presence of shared cross-reactive antigens (Welsh et al., 1980).
2) Allogeneic anti-HLA-B27 antibodies obtained from human tissue typing sera were found to bind to Klebsiella antigens more than other tissue typing sera (Avakian et al., 1980).
3) Anti-HLA-B27 monoclonal antibodies were found to bind more specifically to 60 and 80 Kd components of Klebsiella, whereas no such reactivity was demonstrated by five other monoclonal antibodies (Ogasawara et al., 1986).
4) No significant difference was observed in the immunological reaction between HLA-B27 positive lymphocytes whether obtained from AS patients or healthy controls when treated against sera from rabbits immunized with Klebsiella microbes in comparison to lymphocytes from HLA-B27 negative individuals, which indicates that there are no differences between antigenic specificities of HLA-B27 molecules whether taken from diseased or healthy individuals (Baines et al., 1990).
5) IgA antibody levels against synthetic peptides carrying Klebsiella or HLA-B27 cross-reactive antigens were found to be elevated in sera of Japanese AS patients compared to rheumatoid arthritispatients or healthy controls (Tani et al., 1997a), most probably due to the existing bacterial and self cross-reactive antigens in AS patients.
6) Secretory IgA2 antibodies were found to be significantly increased against type I, III, and IV collagens in sera of Japanese patients with AS when compared to healthy individuals (Tani et al., 1997b).
7) Antibodies from sera of AS patients were found to be cytotoxic to HLA-B27-peptide-bearing cells as shown by increased percentage lysis for sheep red blood cells coated with HLA-B*2705 peptide when compared to patients with rheumatoid arthritis and healthy controls (Wilson et al., 2003), indicating that these autoantibodies probably contribute to the immunological damages which take place at the pathological sites in patients with AS or CD.
Klebsiella Antibodies in AS and CD
During the last three decades extensive studies have been carried out by independent scientific groups using various immunological methods in order to determine the levels of antibodies against Klebsiella and other related enterobacterial microbes in AS patients from 16 different countries. Nearly all these studies showed that antibody levels against Klebsiella antigens (or cross-reactive self-antigens) but not against other microbes have been found to be elevated more significantly in patients with AS when compared to patients with other diseases or to healthy controls (Ebringer et al., 2006; Rashid et al., 2007).
Furthermore, several groups have shown that significantly elevated antibodies to Klebsiellamicroorganisms have been detected in patients with IBD, especially CD, when compared to corresponding healthy controls (Ibbotson et al., 1987; Cooper et al., 1988; O’Mahony et al., 1992; Tiwana et al., 1997; 1998). In a later study, a positive correlation was detected between levels of antibodies against Klebsiella and collagen cross-reactive antigens in patients with CD (Tiwana et al., 2001).
The consistent observation of significantly elevated levels of antibodies against Klebsiella but not other related enterobacterial agents in patients with AS and CD but not in patients with other rheumatic diseases supports the suggestion that this microbe-disease link is not an epiphenomenon but a specific pathological characteristic.
Molecular Mimicry Hypothesis and Pathogenetic Mechanism in AS and CD
Rheumatic fever has been recognized for the last five decades as a systemic arthritic disease caused by early Streptococcal upper respiratory infection with resultant pathological lesions affecting collagens in the joints (Jaggi, 2011), heart muscles (Towers et al., 2009), and brain tissues causing Sydenham’s chorea (Cardoso, 2011). These lesions are caused by the binding of anti-Streptococcal antibodies to the self cross-reactive antigens at these sites. The same pathogenetic mechanism can be involved in the development of AS and CD where anti-Klebsiella antibodies are binding to the cross-reactive self antigens at the entheses of the large joints, especially spinal vertebrae as the result of complement dependent cytopathic autoantibody reactions with self antigens (Figure 3). It would appear that rheumatic fever, AS, and CD are all autoimmune diseases evoked by antibodies to environmental bacteria, the infectious agents being located at different pathological sites. Such chronic autoimmune diseases, however, cannot satisfy Koch’s criteria of an infectious disease.
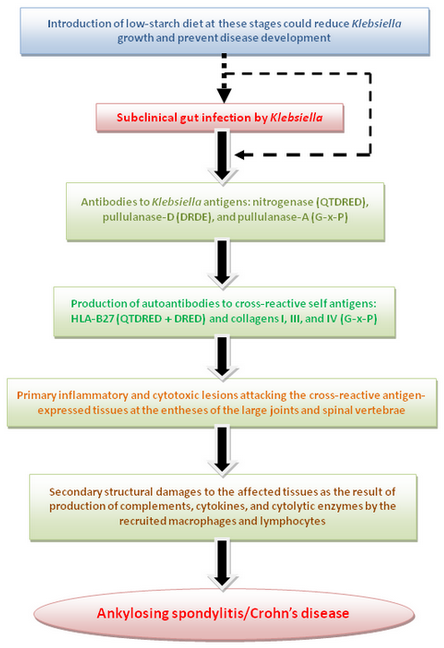
In patients with AS and CD we suggest that Klebsiella infection in the bowel, whether overt or subclinical, primarily causes production of anti-Klebsiella antibodies which can also bind to the cross-reactive self antigens like HLA-B27 and collagen fibers in the joints with release of further new antigens on the surface of damaged tissue. These new antigens are responsible for prolonged or continuous production of autoantibodies and further damages to the articular tissues with a perpetuation in the disease process. Recurrent infections with these bacteria could explain the characteristic remission/exacerbation features which have been observed frequently in patients with these diseases.
The entheses in and around the sacroiliac and spinal joints are the principal sites of inflammation in patients with AS (Hamdi et al., 2011) and CD (Bandinelli et al., 2011). The tendency for the occurrence of pathological lesions at these locations within the territory of the spinal joints is most probably due to their proximity with the draining local lymphatic plexus (Batson’s plexus) from the large bowel in which Klebsiella and other enterobacterial species are abundant.
Proposal for the Use of a New Therapeutic Protocol in AS and CD
The current medical treatment in patients with AS includes the use of non-steroidal anti-inflammatory drugs, immunomosuppresive drugs, and biological agents (Sieper, 2008; Song and Poddubnyy, 2011), whilst in patients with CD in addition to these drugs aminosalicylates, antibiotics, and corticosteroids can also be included in the management (Triantafillidis et al., 2011). Nearly all of these therapeutic modalities, however, are not curative but significantly reduce the degree of inflammation and prolong the duration of remission. Furthermore, most if not all of these treatments have some mild to moderate (Rimbas et al., 2011) or even lethal (Renaud et al., 2011) adverse effects. Nevertheless, it is generally agreed that AS and especially CD are both non-curable diseases and associated with considerably high recurrence rates even with the use of the proper treatments. For example, the recurrence rates in patients with CD have been shown to range between 50% (Colombel et al., 2010) to over 70% (Burt et al., 2010). In a more recent meta-analytical study, it was shown that stopping the treatment with the immunosuppressant, azathioprine, could increase the likelihood of recurrence rate among patients with CD (French et al., 2011).
We suggest that in addition to the currently used medicines other therapeutic modalities could be included in the management of these diseases. One of the main candidates for this new therapeutic protocol is the use of a low starch diet. Klebsiella microbes are considered as one group of bacterial agents that is basically used for the production of ethanol by fermentation. Monosaccharides and disaccharides, which are principally derived from digestion of complex carbohydrates, such as starch, are the main substrate materials available in the gut for microbial growth and survivals. Normal individuals fail to absorb up to 20% of dietary starch materials, which are present in wheat flour products such as bread and pasta, as assessed by oral hydrogen excretion studies, following a test meal (Anderson et al., 1981).
In a study carried out by a group from Los Angeles, it was observed that the mean number of fecal Klebsiella microorganisms in individuals taking high carbohydrate/low protein diet was 40 times higher than in those subjects receiving low carbohydrate/high protein diet (Finegold et al., 1977). In a similar study, the mean number of Klebsiella was found to be 10 times higher for simple sugars (sucrose, lactose, and glucose) per gram of substrate compared to the value obtained after incubation with 11 different amino acids (Ebringer et al., 1985), which indicates that carbohydrates are highly necessary for the growth and replications of Klebsiella or even other related enteropathic microorganisms. In the same study, a significant drop in the erythrocyte sedimentation rate and serum total IgA have been observed by the end of a 9-month period in patients with AS receiving low starch diet. Most patients in the study have reported positive responses from partial to complete recovery from the disease, ranging from disappearance of symptoms to the drop of or even completely stopping the usage of anti-inflammatory drugs. However, the same group of patients did not show any difference in these laboratory parameters when examined retrospectively during the same period of time before they went on the low starch diet.
In the AS patients attending the “London AS Clinic” at the Middlesex Hospital for the past 20 years, it has been found that normally it takes around 6-8 months for the diet to show its effects, and that the majority of AS patients could be treated with dietary manipulation and exercises without the requirements for supplementary pharmaceutical treatments (Ebringer and Wilson, 1996). It is plausible that low starch diet might affect bowel flora by depleting the substrates necessary for enterobacterial growth. For example it could stop or decrease the induction of substrate-dependent microbial products, such as pullulanase, which is a starch debranching enzyme produced by Klebsiellamicroorganisms, with a possibility of having ameliorating effects on the disease outcome.
Based on these data results it is suggested that a low starch diet intake could help in the eradication of Klebsiella and other related enterobacterial species from the bowel and might lead to a decrease in the activity, progress, and full development of AS, CD, and possibly other disease entities of SpAs.
Conclusion
It appears from the results of various independent studies that the pathogenesis of AS and CD involves a genetically-determined, abnormal host immune response to a bacterial stimulus, which is most likely to be Klebsiella. An extensive evidence exists which supports the role for Klebsiellamicrobes in the etiopathogenesis of AS and CD based on the results of various independent microbiological, molecular, and immunological studies.
We suggest that, together with the currently used treatments, inclusion of a low starch dietary intake in patients with AS and/or CD could be beneficial especially when started in the early disease stages and especially in the highly susceptible individuals with positive family history of SpAs or those possessing HLA-B27 genetic allelotypes.
Acknowledgments
This study was supported by the Trustees of the Middlesex Hospital, and the “American Friends of Kings College London.”
Disclosure
The authors declare no conflict of interests.
Corresponding Author
Professor Alan Ebringer, M.D., Analytical Sciences Group, Kings College London, 150 Stamford Street, London SE1 9NN, United Kingdom.
References
Anderson IH, Levine AS, Levitt MD. Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med 304(15):891-892, 1981.
Avakian H, Welsh J, Ebringer A, Entwistle CC. Ankylosing spondylitis, HLA-B27 and Klebsiella. II. Cross-reactivity studies with human tissue typing sera. Br J Exp Pathol 61(1):92-96, 1980.
Baines M, Ebringer A, Avakian H, Samuel D, James DC. The use of enzyme immunoassay (EIA) and radiobinding assay to investigate the cross-reactivity of Klebsiella antigens and HLA-B27 in ankylosing spondylitis patients and healthy controls. Scand J Rheumatol 19(5):341-349, 1990.
Bandinelli F, Milla M, Genise S, Giovannini L, Bagnoli S, Candelieri A, Collaku L, Biagini S, Cerinic MM. Ultrasound discloses entheseal involvement in inactive and low active inflammatory bowel disease without clinical signs and symptoms of spondyloarthropathy. Rheumatology (Oxford) 50(7):1275-1279, 2011.
Braun J, Sieper J. Ankylosing spondylitis, other spondyloarthritides, and related conditions. In: Oxford Textbook of Medicine. Warrell DA, Cox TM, et al. (eds.). pp. 3603-3616. Oxford University Press, Oxford, United Kingdom, 2010.
Brewerton DA, Hart FD, Nicholls A, Cafferty MF, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet 1(7809):904-907, 1973.
Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, Testori A, Halverson A, Verda L, de Villiers WJ, Jovanovic B, Ovama Y. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn Disease: long-term follow-up. Blood116(26):6123-6132, 2010.
Calin A. Reiter’s syndrome-the clinical spectrum. In: The Spondyloarthritides. Calin A and Taurog JD (eds.). pp. 41-57. Oxford University Press, Oxford, United Kingdom, 1998.
Cardoso F. Sydenham’s chorea. Handb Clin Neurol 100:221-229, 2011.
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CI, Rutgeerts P. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 362(15):1383-1395, 2010.
Cooper R, Fraser SM, Sturrock RD, Gemmell CG. Raised titres of anti-Klebsiella IgA in ankylosing spondylitis, rheumatoid arthritis, and inflammatory bowel disease. Br Med J 296(6634):1432-1434, 1988.
Demetter P, Van Huysse J, De Keyser F, Van Damme N, Verbruggen G, Mielants H, De Vos M, Veys EM, Cuvelier CA. Increase in lymphoid follicle and leukocyte adhesion molecules emphasizes a role for the gut in spondyloarthropathy pathogenesis. J Pathol 198(4):517-522, 2002.
Ebringer A, Baines M, Childerstone M, Ghuloom M, Ptaszynska T. Etiopathogenesis of ankylosing spondylitis and the cross-tolerance hypothesis. In: Advances in inflammation research-The spondyloarthropathies. Ziff M and Cohen SB (eds.). pp. 101-128. Raven Press, New York, New York, USA, 1985.
Ebringer A, Wilson C. The use of low starch diet in the treatment of patients suffering from ankylosing spondylitis. Clin Rheumatol 15(Suppl 1):62-66, 1996.
Ebringer A, Rashid T, Wilson C, Ptaszynska T, Fielder M. Ankylosing spondylitis, HLA-B27 and Klebsiella – an overview: proposal for early diagnosis and treatment. Curr Rheumatol Rev 2(1):55-68, 2006.
Ebringer A, Rashid T, Tiwana H, Wilson C. A possible link between Crohn’s disease and ankylosing spondylitis via Klebsiella infections. Clin Rheumatol 26(3):289-297, 2007.
Ebringer A, Rashid T, Fielder M, Wilson C. Ankylosing spondylitis, HLA-B27, Klebsiella and “Popper sequences”. Curr Rheumatol Rev, epub ahead of print, 2011.
Fielder M, Pirt SJ, Tarpey I, Wilson C, Cunningham P, Ettelaie C, Binder A, Bansal S, Ebringer A. Molecular mimicry and ankylosing spondylitis: possible role of a novel sequence in pullulanase of Klebsiella pneumoniae. FEBS Lett 369(2-3):243-248, 1995.
Finegold SM, Sutter VL, Sugihara PT, Elder HA, Lehmann SM, Philips RL. Fecal microbial flora in Seventh Day Adventist populations and control subjects. Am J Clin Nutr 30(11):1781-1792, 1977.
French H, Mark Dalzell A, Srinivasan R, El-Matary W. Relapse rate following azathioprine withdrawal in maintaining remission for Crohn’s disease: A meta-analysis. Dig Dis Sci 56(7):1929-1936, 2011.
Hamdi W, Chelli-Bouaziz M, Ahmed MS, Ghannouchi MM, Kaffel D, Ladeb MF, Kchir MM. Correlations among clinical, radiographic, and sonographic scores for enthesitis in ankylosing spondylitis. Joint Bone Spine 78(3):270-274, 2011.
Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: An animal model of HLA-B27-associated human disorders. Cell 63(5):1099-1112, 1990.
Ibbotson JP, Pease PE, Allan RN. Serological studies in Crohn’s disease. Eur J Clin Microbiol6(3):286-290, 1987.
Jaggi P. Rheumatic fever and post group-a streptococcal arthritis. Pediatr Infect Dis J 30(5):424-425, 2011.
Leirisalo-Repo M. Reactive arthritis. Scand J Rheumatol 34(4):251-259, 2005.
Mau W, Zeidler H, Mau R, Majewski A, Freyschmidt J, Stangel W, Deicher H. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol15(7):1109-1114, 1988.
Mielants H, De Keyser F, Baeten D, Van den Bosch F. Gut inflammation in the spondyloarthropathies. Curr Rheumatol Rep 7(3):188-194, 2005.
Ogasawara M, Kono DH, Yu DTY. Mimicry of human histocompatibility HLA-B27 antigens by Klebsiellapneumoniae. Infect Immun 51(3):901-908, 1986.
O’Mahony S, Anderson N, Nuki G, Ferguson A. Systemic and mucosal antibodies to Klebsiella in patients with ankylosing spondylitis and Crohn’s disease. Ann Rheum Dis 51(12):1296-1300, 1992.
Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J Rheumatol 29(3):511-515, 2002.
Porzio V, Biasi G, Corrado A, De Santi M, Vindigni C, Viti S, Bayeli PF, Marcolongo R. Intestinal histological and ultrastructural inflammatory changes in spondyloarthropathy and rheumatoid arthritis. Scand J Rheumatol 26(2):92-98, 1997.
Rambukkana A, Das PK, Witkamp L, Yong S, Meinardi MM, Bos JD. Antibodies to mycobacterial 65-kDa heat shock protein and other immunodominant antigens in patients with psoriasis. J Invest Dermatol 100(1):87-92, 1993.
Rashid T, Ebringer A. Ankylosing spondylitis is linked to Klebsiella-the evidence. Clin Rheumatol26(6):858-864, 2007.
Rashid T, Ebringer A, Tiwana H, Fielder M. Role of Klebsiella and collagens in Crohn’s disease: A new prospect in the use of low-starch diet. Eur J Gastroenterol Hepatol 21(8):843-849, 2009.
Renaud C, Ovetchkine P, Bortolozzi P, Saint-Cyr C, Tapiero B. Fatal group A Streptococcus pupura fulminans in a child receiving TNF-α blocker. Eur J Pediatr 170(5):657-660, 2011.
Rimbas M, Marinescu M, Voiosu MR, Baicus CR, Caraiola S, Nicolau A, Nitescu D, Badea GC, Parvu MI. NSAID-induced deleterious effects on the proximal and mid small bowel in seronegative spondyloarthropathy patients. World J Gastroenterol 17(8):1030-1035, 2011.
Sany J, Rosenberg F, Panis G, Serre H. Unclassified HLA-B27 inflammatory rheumatic diseases: followup of 23 patients. Arthritis Rheum 23(2):258-259, 1980.
Schattenkirchner M, Kruger K. Natural course and prognosis of HLA-B27-positive oligoarthritis. Clin Rheumatol 6(Suppl 2):83-86, 1987.
Schlosstein L, Terasaki PI, Bluestone E, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med 288(14):704-706, 1973.
Schwimmbeck PL, Yu DTY, Oldstone MBA. Autoantibodies to HLA-B27 in the sera of HLA-B27 patients with ankylosing spondylitis and Reiter’s syndrome: Molecular mimicry with Klebsiellapneumoniae as potential mechanism of autoimmune disease. J Exp Med 166(1):173-181, 1987.
Sieper J. Management of ankylosing spondylitis. In: Rheumatology. Hochberg MC, Silman AJ, et al. (eds.). pp. 1143-1164. Elsevier-Mosby, St. Louis, Missouri, USA, 2008.
Song IH, Poddubnyy D. New treatment targets in ankylosing spondylitis and other spondyloarthritides. Curr Opin Rheumatol 23(4):346-351, 2011.
Tani Y, Tiwana H, Hukuda S, Nishioka J, Fielder M, Wilson C, Bansal S, Ebringer A. Antibodies to Klebsiella, Proteus and HLA-B27 peptides in Japanese patients with ankylosing spondylitis and rheumatoid arthritis. J Rheumatol 24(1):109-114, 1997a.
Tani Y, Sato H, Tanaka N, Mori K, Doida Y, Hukuda S. Serum IgA1 and IgA2 subclass antibodies against collagens in patients with ankylosing spondylitis. Scand J Rheumatol 26(5):380-382, 1997b.
Thomas GP, Brown MA. Genomics of ankylosing spondylitis. Discov Med 10(52):263-271, 2010.
Tiwana H, Wilson C, Walmsley RS, Wakefield AJ, Smith MS, Cox NL, Hudson MJ, Ebringer A. Antibody responses to gut bacteria in ankylosing spondylitis, rheumatoid arthritis, Crohn’s disease and ulcerative colitis. Rheumatol Int 17(1):11-16, 1997.
Tiwana H, Walmsley RS, Wilson C, Yiannakou JY, Ciclitira PJ, Wakefield AJ, Ebringer A. Characterization of the humoral immune response to Klebsiella species in inflammatory bowel disease and ankylosing spondylitis. Br J Rheumatol 37(5):525-531, 1998.
Tiwana H, Natt RS, Benitez-Brito R, Shah S, Wilson C, Bridger S, Harbord M, Sarner M, Ebringer A. Correlation between the immune responses to collagens type I, III, IV and V and Klebsiella pneumoniae in patients with Crohn’s disease and ankylosing spondylitis. Rheumatology (Oxford)40(1):15-23, 2001.
Towers RJ, Bolm M, Currie BJ, Chhatwal GS, Fagan PK. Autoantigens identified by screening a human heart cDNA library with acute rheumatic fever sera. Ann N Y Acad Sci 1173:83-91, 2009.
Townes JM. Reactive arthritis after enteric infections in the United States: the problem of definition. Clin Infect Dis 50(2):247-254, 2010.
Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther 5:185-210, 2011.
Uppal SS, Abraham M, Chowdhury RI, Kumari R, Pathan EM, Al-Rashed A. Ankylosing spondylitis and undifferentiated spondyloarthritis in Kuwait: a comparison between Arabs and South Asians. Clin Rheumatol 25(2):219-224, 2006.
Welsh J, Avakian H, Cowling P, Ebringer A, Wooley P, Panayi G, Ebringer R. Ankylosing spondylitis, HLA-B27 and Klebsiella. I. Cross-reactivity studies with rabbit antisera. Br J Exp Pathol 61(1):85-91, 1980.
Wilson C, Rashid T, Tiwana H, Beyan H, Hughes L, Bansal S, Ebringer A, Binder A. Cytotoxicity responses to peptide antigens in rheumatoid arthritis and ankylosing spondylitis. J Rheumatol30(5):972-978, 2003.
Wright V. Seronegative polyarthritis: a unified concept. Arthritis Rheum 21(6):619-633, 1978.
[Discovery Medicine; ISSN: 1539-6509; Discov Med 12(64):187-194, September 2011. Copyright © Discovery Medicine. All rights reserved.]